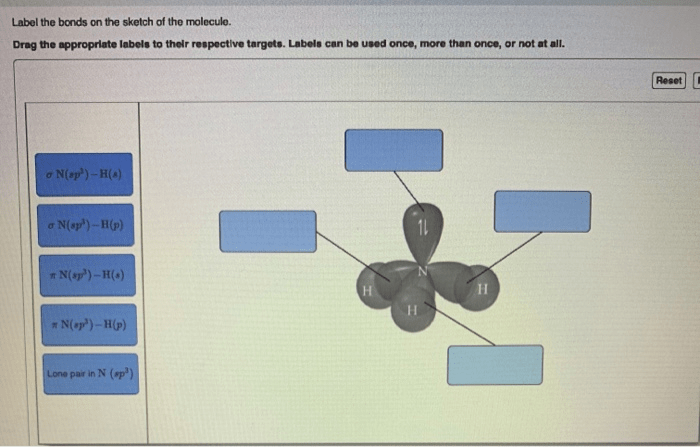
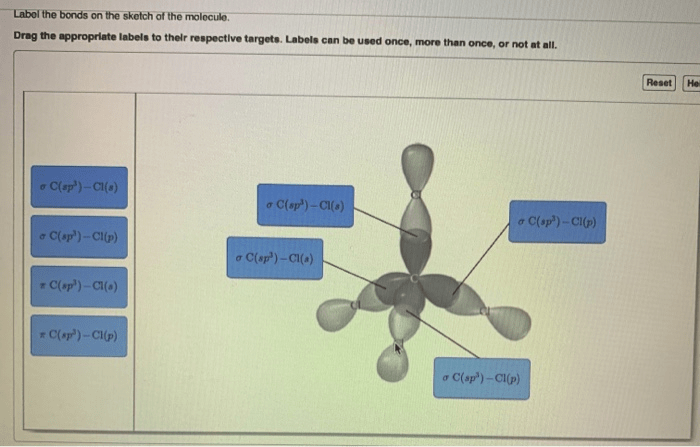
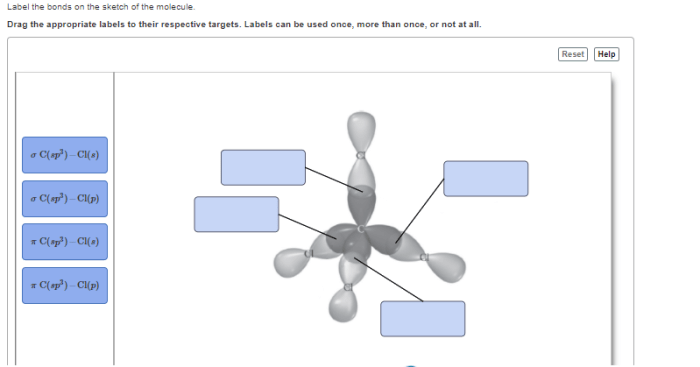
Label the bonds on the sketch of the molecule. – Labeling the bonds on a molecular sketch is a fundamental aspect of chemistry, providing insights into the molecular structure, bonding characteristics, and reactivity. This guide will delve into the intricacies of bond labeling, encompassing bond types, bond order, structural representation, resonance, and exceptions to the typical labeling rules.
Understanding bond labeling empowers chemists to accurately depict molecular structures, predict molecular properties, and unravel the mechanisms of chemical reactions.
Labeling Bond Types
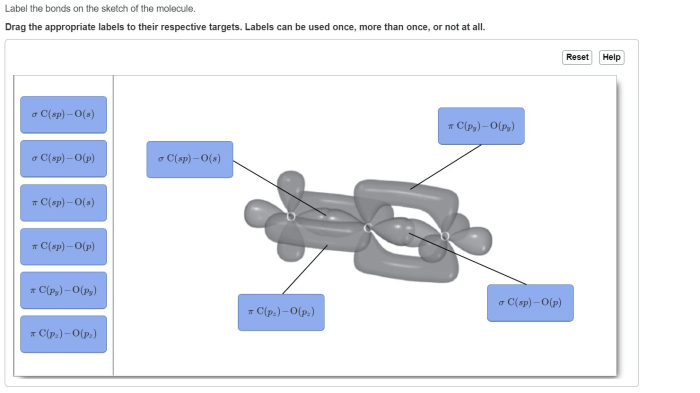
Understanding the different types of bonds and how they are labeled is crucial in chemistry. This article aims to provide a comprehensive overview of bond types, labeling bond order, structural representation, resonance and bond labeling, and exceptions and special cases.
Identifying Bond Types
Chemical bonds are classified based on the type of overlap between atomic orbitals. There are two main types of bonds:
- Sigma (σ) bonds: Formed by head-to-head overlap of orbitals, resulting in a cylindrical electron density around the bond axis.
- Pi (π) bonds: Formed by lateral overlap of orbitals, resulting in electron density above and below the bond axis.
The number of shared electron pairs determines the bond order, which in turn affects bond strength and length:
- Single bond: One sigma bond, with one shared electron pair.
- Double bond: One sigma bond and one pi bond, with two shared electron pairs.
- Triple bond: One sigma bond and two pi bonds, with three shared electron pairs.
Labeling Bond Order
Bond order is labeled based on the number of shared electron pairs:
- Single bond: Bond order 1
- Double bond: Bond order 2
- Triple bond: Bond order 3
Bond order can be determined by counting the number of shared electron pairs or by examining the molecular orbital diagram.
Structural Representation, Label the bonds on the sketch of the molecule.
Structural representation provides a visual depiction of bonds and molecular structure. Two common methods are:
- Lewis structures: Show the connectivity of atoms and the number of valence electrons.
- Molecular orbital diagrams: Show the distribution of electrons in molecular orbitals.
These representations help visualize bond types and molecular geometry.
Resonance and Bond Labeling
Resonance occurs when a molecule has multiple valid Lewis structures. In such cases, the actual structure is a hybrid of these resonance contributors.
- Resonance contributors: Individual Lewis structures that contribute to the overall resonance hybrid.
In resonance structures, bonds may be labeled as:
- Single bond: Bond order 1
- Double bond: Bond order 1.5
- Triple bond: Bond order 1.33
Exceptions and Special Cases
Some exceptions to the typical bond labeling rules exist:
- Hyperconjugation: Overlap between a sigma bond and a pi bond, resulting in increased bond order.
- Aromaticity: Resonance in cyclic molecules with alternating single and double bonds, resulting in increased bond stability.
Query Resolution: Label The Bonds On The Sketch Of The Molecule.
What is the difference between a sigma bond and a pi bond?
A sigma bond is formed by the head-to-head overlap of atomic orbitals, while a pi bond is formed by the lateral overlap of atomic orbitals.
How do you determine the bond order of a bond?
Bond order is determined by the number of shared electron pairs between the bonded atoms.
What is resonance, and how does it affect bond labeling?
Resonance occurs when a molecule has multiple valid Lewis structures, and it affects bond labeling by delocalizing the electrons in the bonds, resulting in partial bond orders.