HCO3- bonding and lone pairs play a crucial role in shaping the molecular structure, properties, and reactivity of this important species. Delve into this captivating exploration to uncover the intricacies of its chemical makeup and its significance in various scientific disciplines.
HCO3- is a fascinating molecule composed of one carbon atom, three oxygen atoms, and one hydrogen atom. The carbon atom forms three covalent bonds with the oxygen atoms, resulting in a trigonal planar geometry. The hydrogen atom is attached to one of the oxygen atoms, completing the molecular structure.
HCO3- Molecular Structure
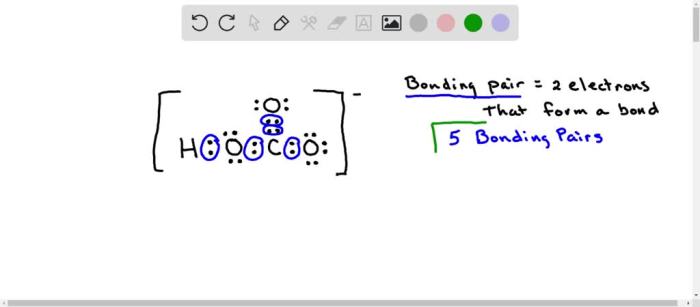
The hydrogen carbonate ion (HCO3-) is a polyatomic ion with the chemical formula HCO3-. It is composed of one hydrogen atom, one carbon atom, and three oxygen atoms. The carbon atom is bonded to the three oxygen atoms by single bonds, and the hydrogen atom is bonded to one of the oxygen atoms by a single bond.
The molecular structure of HCO3- is trigonal planar, with the carbon atom at the center and the three oxygen atoms arranged in a plane around it. The hydrogen atom is located above the plane of the three oxygen atoms.
Bonding in HCO3-
The bonding in HCO3- can be described using valence bond theory. The carbon atom has four valence electrons, and each oxygen atom has six valence electrons. The carbon atom forms single bonds with each of the three oxygen atoms by sharing two electrons with each oxygen atom.
The hydrogen atom forms a single bond with one of the oxygen atoms by sharing two electrons with the oxygen atom. The resulting Lewis structure of HCO3- is:“`O=C-O-H“`The HCO3- ion has a net negative charge because it has one more electron than the number of protons in the nucleus.
The negative charge is delocalized over the three oxygen atoms, which means that each oxygen atom has a partial negative charge.
Bonding in HCO3-
The HCO3- ion is a polyatomic ion composed of one carbon atom, three oxygen atoms, and one hydrogen atom. It is a resonance hybrid, meaning that the electrons in the molecule are delocalized over several atoms. This results in the formation of two types of bonds: covalent bonds and coordinate bonds.
Types of Bonds in HCO3-
The HCO3- ion contains two types of covalent bonds: a single bond between the carbon atom and the oxygen atom that is bonded to the hydrogen atom, and two double bonds between the carbon atom and the other two oxygen atoms.
The single bond is formed by the overlap of one electron from the carbon atom and one electron from the oxygen atom. The double bonds are formed by the overlap of two electrons from the carbon atom and two electrons from each oxygen atom.
In addition to the covalent bonds, the HCO3- ion also contains one coordinate bond. A coordinate bond is a type of covalent bond in which one atom donates both electrons that form the bond. In the HCO3- ion, the hydrogen atom donates both electrons that form the coordinate bond with the oxygen atom that is bonded to the carbon atom.
Hybridization of the Carbon Atom and Geometry of the Molecule
The carbon atom in the HCO3- ion is sp2 hybridized. This means that the carbon atom has three hybrid orbitals that are formed by the overlap of one s orbital and two p orbitals. The three hybrid orbitals are arranged in a trigonal planar geometry.
The oxygen atoms that are bonded to the carbon atom are located at the corners of the triangle, and the hydrogen atom is located above the plane of the triangle.
Lone Pairs in HCO3-
The oxygen atoms in HCO3- possess lone pairs, which significantly influence the molecular structure and properties of the ion.
Number of Lone Pairs
Each oxygen atom in HCO3- has two lone pairs of electrons, resulting in a total of six lone pairs. These lone pairs are located on the oxygen atoms that are not bonded to hydrogen atoms.
Effect on Molecular Structure
The lone pairs on the oxygen atoms repel each other, causing the HCO3- ion to adopt a trigonal planar molecular structure. The carbon atom is at the center of the triangle, with the three oxygen atoms arranged around it. The lone pairs occupy the remaining three corners of the triangle, pushing the oxygen atoms apart and giving the ion its characteristic shape.
Effect on Properties, Hco3- bonding and lone pairs
The lone pairs on the oxygen atoms also affect the properties of HCO3-. For instance, they contribute to the ion’s polarity, as the lone pairs create a partial negative charge on the oxygen atoms. Additionally, the lone pairs can participate in hydrogen bonding, allowing HCO3- to form interactions with other molecules.
Resonance in HCO3-
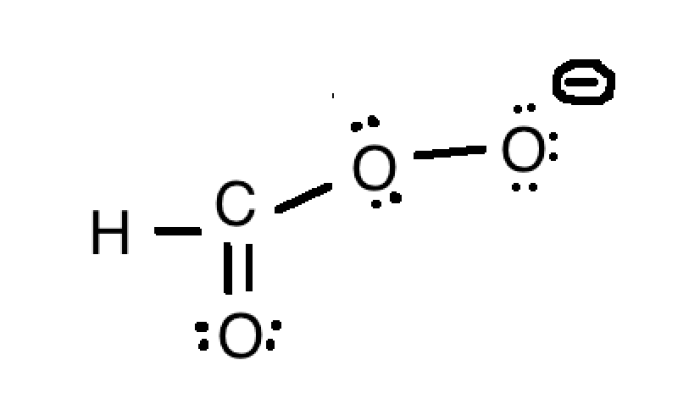
The HCO3- ion, also known as the bicarbonate ion, is a polyatomic ion that plays a vital role in many biological processes. It is a resonance hybrid, meaning that its structure can be represented by several resonance structures. Resonance is a phenomenon that occurs when a molecule or ion has multiple Lewis structures with the same number of valence electrons.
The bonding and lone pairs of HCO3- play a significant role in its chemistry. If you need a refresher on this topic, check out the mid unit 1 review limits . This resource provides a comprehensive overview of the concepts surrounding HCO3- bonding and lone pairs.
In the case of HCO3-, the three resonance structures are shown below:
- O=C-O –
- –O-C-O
- O-C –-O
These resonance structures show that the negative charge can be delocalized over the three oxygen atoms, which results in a more stable molecule. The delocalization of the negative charge also affects the properties of the molecule, such as its acidity and basicity.
Effect of Resonance on Stability
The resonance in HCO3- contributes to its stability by delocalizing the negative charge over the three oxygen atoms. This delocalization reduces the electron density on any one oxygen atom, which makes the molecule less reactive and more stable.
Effect of Resonance on Properties
The resonance in HCO3- also affects its properties, such as its acidity and basicity. The delocalization of the negative charge makes the molecule less acidic and more basic. This is because the negative charge is less likely to be donated to another molecule, which makes the molecule less acidic.
The delocalization of the negative charge also makes the molecule more likely to accept a proton, which makes it more basic.
Acid-Base Properties of HCO3-

HCO3- is an amphoteric ion, meaning it can act as both an acid and a base. As an acid, it can donate a proton (H+) to form H2CO3. As a base, it can accept a proton to form CO32-. The acid-base properties of HCO3- are important in biological systems, where it plays a role in buffering the pH of blood and other fluids.
Role as a Buffer in Biological Systems
A buffer is a solution that resists changes in pH when small amounts of acid or base are added. HCO3- is a major component of the bicarbonate buffer system in the blood, which helps to keep the pH of blood within a narrow range (7.35-7.45).
When the pH of blood decreases (becomes more acidic), HCO3- can donate protons to form H2CO3, which helps to neutralize the acid and bring the pH back up. Conversely, when the pH of blood increases (becomes more basic), HCO3- can accept protons to form CO32-, which helps to neutralize the base and bring the pH back down.
Query Resolution: Hco3- Bonding And Lone Pairs
What is the hybridization of the carbon atom in HCO3-?
The carbon atom in HCO3- is sp2 hybridized.
How many lone pairs are present on the oxygen atoms in HCO3-?
There are three lone pairs present on the oxygen atoms in HCO3-.
What is the role of HCO3- as a buffer in biological systems?
HCO3- plays a crucial role in maintaining the pH balance of blood and other bodily fluids.